Home / Healthcare
Healthcare
At Avesthagen Limited, we are developing cost effective Biological drugs for large therapeutic markets, with an immediate term focus on an emerging global consumer class in the BRIC countries and other countries with emerging markets.
Biological drugs are those that have an active ingredient that is made from a living organism. They are highly complex macromolecules that include recombinant proteins, therapeutic monoclonal antibodies and genetically engineered vaccines. Given their size, biological drugs are usually administered by subcutaneous, intravenous or intramuscular injection unlike traditional small molecule drugs that are typically administered orally.
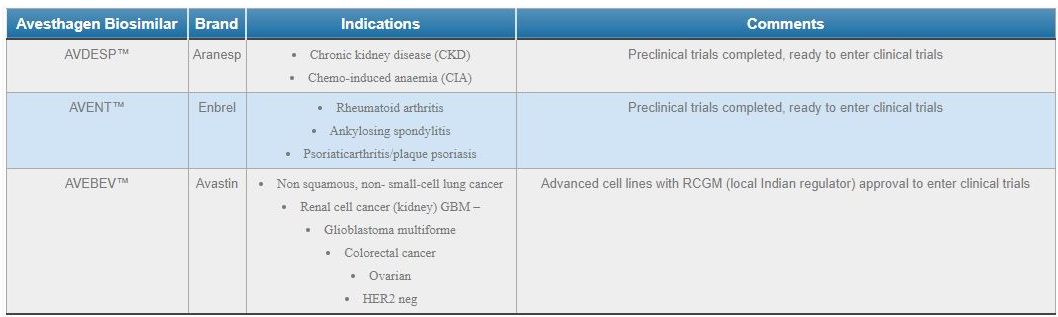
Avesthagen Biopharmaceuticals develops a portfolio of biosimilar products including therapeutics in cancer and autoimmune disorders. Two of these biosimilars, AVDESPTM (for anaemia caused by chronic kidney disease or chemotherapy) and AVENTTM (for rheumatoid arthritis) are ready to enter clinical trials, with three more in advanced stages of development. The Company is looking for long-term partners who will provide development and clinical trials and marketing support.
“I can’t show you how, exactly, healthcare is a basic human right. But what I can argue is that no one should have to die of a disease that is treatable.”
– Paul Farmer
Research and Development
Our bioPharmaceuticals business is focused on the development of a comprehensive biosimilar portfolio of cost-effective and clinically validated biologics-based and antibody therapeutics, for which the originators’ patents are not valid in India under the WTO TRIPS Agreement thereby allowing potential marketing within these markets.
Biosimilars, also known as follow-on biologics or follow-on proteins, are drugs that have a high level of similarity to existing biologics. They have the same qualitative and quantitative composition as the reference biological drug but are not equivalent due to differences in the raw materials used, the manufacturing process and the molecular characteristics.
The biosimilars market is highly attractive given the size, profitability, reimbursement and pricing environment of the underling biological drug industry. Our strategy is to bring this portfolio of molecules to the market, with our partners, with an initial focus on the emerging countries of India, China, Russia and Brazil, and a more long term focus on larger markets in North America and Europe.
Development Process
We have developed biosimilar versions of molecules, the anticipated markets for which are oncology, cardiovascular and arthritis targeted biologics. Upon establishing a proof of concept for a specific drug, our development of a biosimilar molecule involves the following stages:
Preliminary process development
- Upstream process development comprising the construction and verification of host-system specific recombinant protein expression, development of stable cell lines expressing the recombinant protein of interest and the establishment of basal fermentation parameters, preferably in serum-free growth conditions
- Establishment of product specific bioassays to determine recombinant protein product functionality at an early stage
- Design and development of basal recombinant protein product recovery procedures
- Physico-chemical equivalence of the expressed and purified recombinant protein product to the approved originator’s product as the reference control
Establishment of commercially viable yields at upstream recombinant protein production level and downstream product recovery stage
- Selection of producer cell-lines expressing the recombinant protein product of interest and establishment of long-term master cell banks and short-term working cell bank
- Optimization of fermentation process based on the following vital parameters, including, growth media composition, feed strategy development based on nutrient consumption, protein expression inducers and scalability analysis
- Comparative analysis of pre-chromatographic operative procedures such as clarification and sterile filtration
- Screening and optimization of selected chromatographic matrices
- Optimization of intermediate ultra-filtration and dia-filtration procedures
- Optimization of virus and endotoxin removal procedures at the scale down level; and
- Process validation of all the steps mentioned above
Extensive documentation of product-specific bioactivity and in-process quality control (IPQC) assays as standard operating procedures. Comprehensive process validation and batch reproducibility establishment in line with country or region-specific regulatory requirements including scale down virus and endotoxin removal procedures.
Market Significance
Our Biosimilar portfolio is the subject of a current B2B licensing programme with Indian and global Pharma companies. The Biosimilar Market is expected to exceed more than US$ 23.50 Billion by 2024 at a CAGR of 31% in the given forecast period. The pharmaceuticals market in India is estimated to be c.$55 billion by 2020, representing a CAGR of over 22% according to a GlobalData study. One of the main drivers of India’s pharmaceutical sector is the Biosimilars market, which is expected to increase to c.$40 billion globally by 2020 driven by the onset of biologic treatments introduced for diseases such as diabetes, cancer, multiple sclerosis, and rheumatoid arthritis.
The current COVID 19 pandemic is expected to put additional stress on the healthcare market. Hence, the use of biosimilars is among the main resources that governments can use to generate significant savings. Before the pandemic was declared, biosimilars were already key to keeping spending within acceptable levels, but now their need is even more imperative.
Products
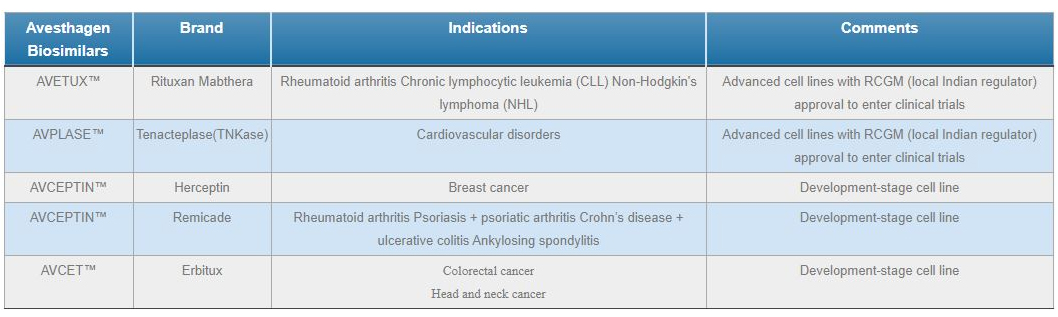
Our bioPharmaceutical product candidates include a large portfolio of Biosimilars, of which we have identified 8 as promising targets for commercialisation. The anticipated markets for which are oncology, cardiovascular and arthritis targeted biologics.
Intellectual Property and Publications
Avesthagen Limited has built a strong IP Portfolio over the last 18 years with a large number of granted patents, trademarks, copyrights and designs registered across the globe. These patents are spread across Healthcare, Wellness & Nutrition, and Environment & Agriculture domains.
Avesthagen is vigilant about protecting and maintaining its invention recognising that its IPR portfolio is of paramount importance for its commercialisation programmes
Avesthagen’s Biosimilar Pipeline in full compliance with the TRIPS-WTO requirement. In the biopharmaceutical portfolio, there are 83 patents at various stages, wherein 2 patents belong to AVENT™ and other 2 patents belong to AVPLASE™ and AVETUX™
 Publications
Publications

